Biologic drugs aren’t like the pills you pick up at the pharmacy. You can’t just reverse-engineer them, mix a few chemicals, and get the same result. That’s because they’re made from living cells - not chemicals. And that tiny difference changes everything.
What Makes Biologics So Different?
Think of a generic aspirin. It’s a simple molecule, small enough to fit in your palm. Chemists can build it step by step, exactly the same way, every time. A batch made in 2020 is identical to one made in 2026. That’s why generics are cheap and easy to copy.
Now think of a biologic drug like Humira or Ozempic. These aren’t molecules - they’re proteins, sometimes thousands of times bigger than aspirin. They’re grown inside living cells - hamster ovary cells, yeast, or bacteria - that have been genetically tweaked to produce the exact protein your body needs. Even then, no two batches are ever perfectly the same. Why? Because living systems aren’t machines. They react to tiny changes in temperature, nutrients, or even the air in the room.
The FDA says it plainly: ‘Slight modifications… are expected as a natural process of manufacturing.’ That’s not a flaw - it’s how biology works. You can’t control every variable in a cell’s environment. And even if you could, we still don’t have the tools to measure every single detail of these massive, complex molecules. Current tests can only confirm about 60-70% of a monoclonal antibody’s structure. The rest? We infer it. That’s why no one can make an exact copy.
How Are Biologics Actually Made?
It’s not a lab experiment. It’s a months-long, million-dollar operation.
First, scientists insert a human gene into a host cell - say, a Chinese hamster ovary cell - so it starts producing the therapeutic protein. Then, those cells are placed in giant bioreactors, like industrial bathtubs filled with nutrient-rich broth. They grow for 10-14 days, kept at exactly 36.5°C, with pH levels held steady. Any drop in oxygen or spike in waste products? The cells start dying. And if the cells die, the whole batch is ruined.
Then comes purification. The protein must be pulled out of the soup of dead cells, leftover nutrients, and viruses. That means passing it through protein A chromatography columns - a fancy filter that grabs only the right protein. Then viral filters, ultrafiltration, buffer swaps. Each step reduces yield. Only about 10-20% of the original protein survives the process.
And it all takes 3-6 months. Compare that to a generic pill, which can be made in days. The cost? Up to $500 million to build a single facility. Failure rates? 10-15% per batch. One contaminated bioreactor can cost over half a million dollars. That’s not just expensive - it’s risky.
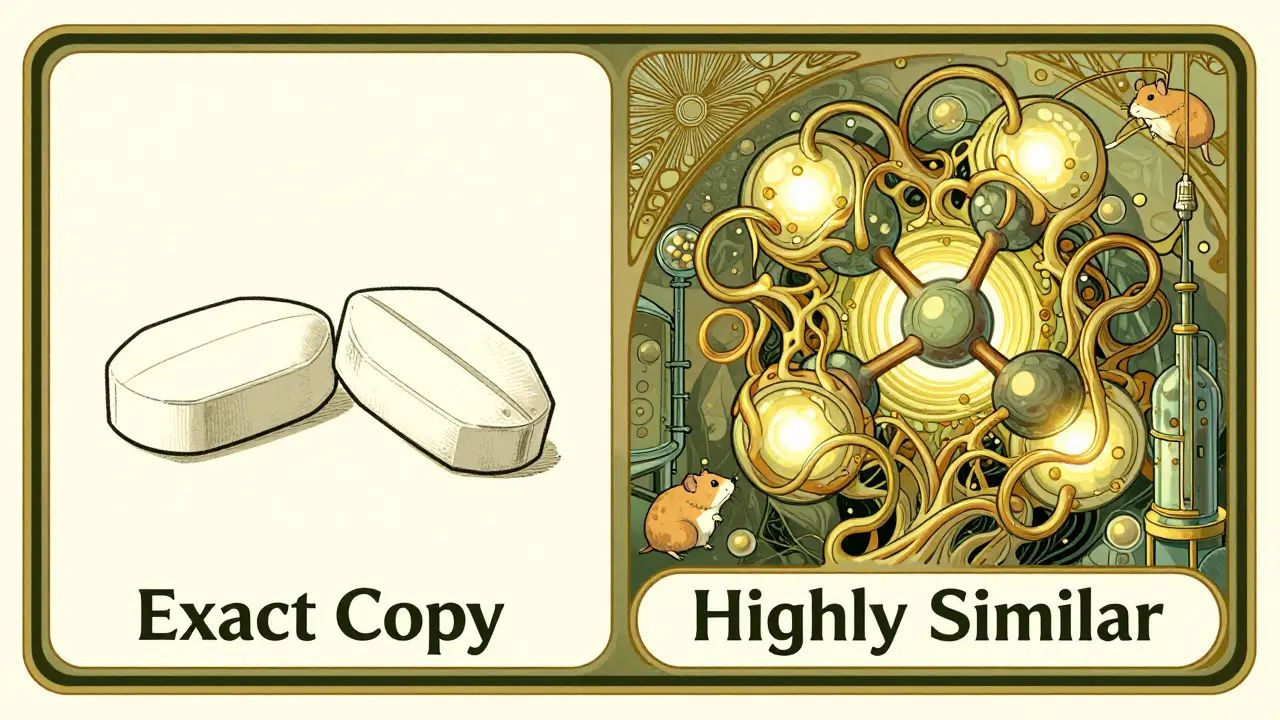
Why Can’t We Just Copy Them Like Generics?
Generics are copies. Biologics are imitations - and even those are hard to get right.
The FDA doesn’t call them generics. They’re called biosimilars. And the difference isn’t just semantics. A generic version of metformin has the same active ingredient, same shape, same effects. A biosimilar? It’s highly similar - but not identical. It has to match the original drug in structure, function, and clinical effect - but tiny differences are allowed, as long as they don’t change safety or effectiveness.
That’s why biosimilars require years of testing. You can’t just run a quick bioequivalence study like you do for pills. You need:
- Thousands of analytical tests comparing molecular structure
- Animal studies to check immune reactions
- Phase 3 clinical trials to prove it works the same in patients
And even then, regulators demand proof that switching from the original to the biosimilar won’t cause unexpected side effects. That’s why it takes 7-10 years and $100-200 million to bring one to market - compared to $1-5 million for a generic.
Some experts argue the bar is too high. Dr. Almut Winterstein from the University of Florida says we’re over-testing minor differences that don’t matter clinically. But regulators won’t budge. Why? Because biologics can trigger immune responses. A tiny change in sugar molecules attached to the protein - something we can’t even fully measure - might cause a patient to develop antibodies that attack not just the drug, but their own body.
The Real Cost of Complexity
It’s not just money. It’s time, talent, and infrastructure.
Biologics manufacturing requires engineers who understand biology, biologists who understand engineering, and quality teams who live in cleanrooms. ISO Class 5 environments - cleaner than operating rooms - are standard. One speck of dust can ruin a batch. Workers wear full-body suits. Air flows in one direction. Every valve, every pipe, every sensor is logged.
Documentation is insane. One product can generate over 10,000 pages of records - every temperature reading, every batch number, every test result. The FDA doesn’t just want to see the final product. They want to see how you got there.
And scaling up? It’s brutal. One engineer at Amgen said switching from a 2,000-liter bioreactor to a 15,000-liter one took 17 months and $22 million in lost revenue. Why? Because the way cells behave in a small tank is not the same as in a huge one. You can’t just scale linearly. You have to re-optimize everything.
That’s why only a handful of companies worldwide can make these drugs. And why biosimilars are still rare - only about 10% of the biologics market is covered by them today, even though patents are expiring.

What’s Changing in the Industry?
Things are starting to shift.
More companies are using single-use bioreactors - plastic bags instead of stainless steel tanks. That cuts cleaning time and cross-contamination risk by 60%. But it raises material costs by 15-20%. It’s a trade-off.
Artificial intelligence is helping too. Some manufacturers now use AI to predict how small changes in temperature or nutrient mix will affect protein quality - before they even run the batch. The FDA’s Emerging Technology Program has helped 47% of early adopters cut manufacturing time by 20-30%.
And the future? Modular factories. Think of them like LEGO biologics plants - you can add or remove units as demand changes. That could slash capital costs by 25-30% and make biosimilars more accessible.
But the biggest hurdle remains: we still can’t fully measure what we’re making. Dr. R. Lou Sherman of the Alliance for Advanced Biologics put it bluntly: ‘Current analytical methods can characterize only 60-70% of a typical monoclonal antibody’s structural attributes.’ That means we’re making life-saving drugs with blind spots. We trust the process. But we don’t fully understand it.
Why This Matters for Patients
Biologics treat diseases that used to be death sentences - rheumatoid arthritis, Crohn’s disease, certain cancers, type 2 diabetes. They’re expensive. Humira cost over $70,000 a year before biosimilars came along.
Biosimilars have started to bring prices down. In Europe, some biosimilars cost 30-50% less. In the U.S., prices are falling slower, but they’re falling. A biosimilar version of adalimumab (Humira) now sells for around $40,000 a year - still high, but half the original.
And that’s the point. You don’t need an exact copy to get the same result. You need a version that’s proven to work the same. The science says it’s possible. The regulators say it’s safe. The market is proving it’s sustainable.
Biologics aren’t copyable. But they’re becoming more accessible. And that’s the real win.
Can a biosimilar be substituted for the original biologic without a doctor’s approval?
In most cases, no. Even though biosimilars are highly similar, they’re not considered ‘interchangeable’ unless they’ve passed extra testing to prove switching back and forth won’t affect safety or effectiveness. Only a few biosimilars have received this designation from the FDA. Most require a doctor to specifically prescribe the biosimilar - just like the original.
Why are biologics so much more expensive than regular drugs?
Because they’re not made in a lab with chemicals - they’re grown in living cells. The process takes months, requires ultra-clean environments, involves complex purification, and has a high failure rate. Building a manufacturing facility costs $100-500 million. Quality control alone can take up to 40% of the total cost. Generics? They’re made in days with simple chemistry.
Are biosimilars safe?
Yes. Every biosimilar must undergo rigorous testing - thousands of analytical comparisons, animal studies, and clinical trials - to prove it works just like the original. Regulatory agencies like the FDA and EMA require proof of no meaningful differences in safety or effectiveness. Millions of patients worldwide have used biosimilars without increased risk.
What’s the difference between a biosimilar and a generic drug?
Generics are exact copies of small-molecule drugs - simple chemicals you can fully analyze and reproduce. Biosimilars are highly similar versions of complex biologics made from living cells. You can’t make them identical because living systems introduce natural variation. That’s why biosimilars need more testing and can’t be called generics.
Will biologics ever become as cheap as generics?
Not in the same way. They’ll get cheaper, but never as cheap as aspirin. The manufacturing complexity, infrastructure, and regulatory burden will always keep costs higher. But with biosimilars, modular factories, and AI-driven production, prices are already dropping - and will continue to fall. The goal isn’t to match generic prices. It’s to make life-saving drugs affordable without compromising safety.







Robin Williams
January 12, 2026 AT 16:38biologics are like trying to clone a soul, man. you can copy the recipe but the magic? that’s in the vibes. cells ain’t robots, they got moods, man. one day they’re chill, next day they’re like ‘nah i quit’ and boom - batch gone. we think we’re smart but we’re just babysitting living things with spreadsheets.
Anny Kaettano
January 13, 2026 AT 16:41the complexity here is staggering - we’re talking about protein folding dynamics, post-translational modifications, glycosylation heterogeneity, and upstream/downstream process variability all converging in a single therapeutic. it’s not just manufacturing - it’s bioengineering art. and yeah, the FDA’s cautious for good reason: immune responses to minor glycan shifts can trigger neutralizing antibodies. this isn’t aspirin.
Damario Brown
January 14, 2026 AT 02:48lol so we spend 500mil to make a drug we dont even fully understand? classic. they dont know what 30% of the molecule does but they inject it into people? why dont we just give them a prayer and call it a day? also typo: bioreactor not biorectar. fix it.
sam abas
January 15, 2026 AT 16:42you say biosimilars are hard to make but you ignore that the original manufacturers deliberately make it hard to copy - patent thickets, data exclusivity, legal bullying. the science isn’t the barrier, it’s the business model. if you gave away the cell line and process specs, 50 countries could make these tomorrow. but then profits drop. so we pretend biology is too complex. it’s not. it’s capitalism.
Clay .Haeber
January 16, 2026 AT 00:15so let me get this straight - we’ve got scientists in hazmat suits whispering sweet nothings to hamster cells like they’re petting a unicorn, all so rich people don’t have to pay $70k/year for a drug that’s basically a protein love letter from a hamster? i’m not mad. i’m just impressed. also, can we call these ‘bio-artisanal’? because that’s what this is. artisanal suffering.
Priyanka Kumari
January 16, 2026 AT 12:35the real win isn’t just lower prices - it’s access. in India, a biosimilar for rheumatoid arthritis costs less than a month’s rent for many families. this isn’t just science - it’s justice. we need more transparency, more collaboration, and less corporate gatekeeping. biology doesn’t care about patents. humans should care more.
Avneet Singh
January 18, 2026 AT 08:54the entire biosimilar paradigm is a regulatory placebo. you can’t prove equivalence when you can’t even characterize 70% of the molecule. it’s like saying two snowflakes are identical because they’re both white. the FDA is just playing along to keep the pharma gravy train rolling. stop calling it science - it’s theater.
Adam Vella
January 18, 2026 AT 12:22It is axiomatic that the biological nature of these therapeutics introduces inherent variability, which is fundamentally incompatible with the paradigm of chemical generics. The notion of ‘exact replication’ is a reductionist fallacy when applied to macromolecular entities derived from eukaryotic expression systems. Therefore, the current regulatory framework, while burdensome, is epistemologically necessary.
Nelly Oruko
January 18, 2026 AT 17:54we make drugs we dont fully understand. that’s wild. but also… kinda beautiful? like we’re holding hands with nature and saying ‘please don’t kill us’.
vishnu priyanka
January 18, 2026 AT 23:55in india, we call these ‘living medicines’ - because they’re not just chemicals, they’re alive. our grandmas say, ‘if it breathes, don’t try to copy it’. turns out, they were right. also, chai helps with the side effects. just saying.
Angel Tiestos lopez
January 20, 2026 AT 21:29cells = tiny little artists 🎨
bioreactors = their studio 🏭
batch failure = their meltdown 😭
we’re just lucky they don’t quit and start a band.
Alan Lin
January 21, 2026 AT 06:49you people are missing the point. this isn’t about science - it’s about accountability. if you can’t fully characterize the product, you have no right to inject it into humans. the FDA should shut down all biologics until we have full structural mapping. This is not acceptable. Millions of patients are being used as test subjects for corporate laziness. I’m not exaggerating. This is dangerous.
mike swinchoski
January 22, 2026 AT 03:58why do we even need this? just give people aspirin and pray. if they die, they were weak anyway. also, hamster cells? that’s just gross. i’m not injecting hamster juice.
Trevor Whipple
January 23, 2026 AT 09:14yo so if we can’t copy it why is humira so expensive? because the company owns the hamster? lol. also i saw a video of a bioreactor and it looked like a sci-fi bathtub. who lets this happen? also typo: bioreacter.
Lethabo Phalafala
January 24, 2026 AT 07:28I’ve watched my sister fight Crohn’s for 12 years. She got her first biologic in 2018. It saved her life. Now, her biosimilar costs half as much. I don’t care if it’s not identical. I care that she’s alive. That’s the only math that matters. Don’t let your cynicism erase someone’s miracle.