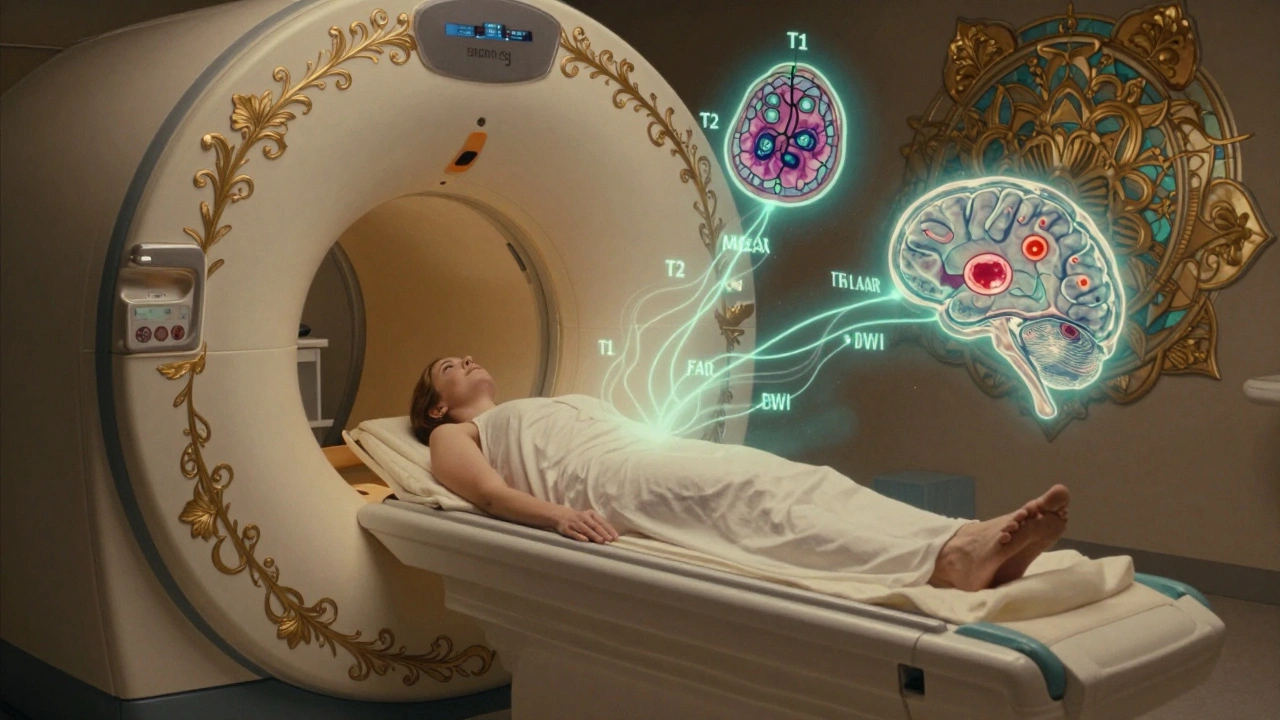
When your doctor orders a brain MRI, it’s not because they’re being overly cautious-it’s because they need to see what’s happening inside your skull with precision. Unlike X-rays or CT scans, MRI doesn’t use radiation. Instead, it uses powerful magnets and radio waves to create detailed pictures of your brain’s soft tissues. This makes it the best tool for spotting problems like tumors, strokes, multiple sclerosis plaques, or even tiny bleeds that other scans might miss. If you’ve been told you need one, you’re probably wondering: what are they looking for? And what do those strange black-and-white images actually mean?
Why MRI Over Other Scans?
Think of a CT scan as a quick snapshot. It’s fast, great for broken bones or fresh bleeding, and often the first thing used in emergency rooms after a head injury. But when it comes to seeing subtle changes in brain tissue-like early signs of dementia, inflammation, or tiny areas of damaged nerve fibers-CT falls short. That’s where MRI comes in.
Brain MRI gives you about 100 times better contrast between gray matter, white matter, and fluid than CT. This means doctors can see the difference between healthy tissue and something abnormal, even if it’s only a few millimeters wide. For example, a small multiple sclerosis lesion might show up clearly on an MRI but look completely normal on a CT. The same goes for early-stage strokes. While CT might not show anything for hours after symptoms begin, MRI can detect changes within minutes using a special sequence called DWI.
Another big advantage? No radiation. That’s why MRI is preferred for kids, pregnant women, and people who need repeat scans over time-like those managing multiple sclerosis or monitoring a brain tumor after treatment.
What Do the Different MRI Sequences Show?
Not all brain MRI images look the same. That’s because radiologists use different “sequences” to highlight different things. Each one works like a filter, making certain tissues shine or fade based on how water behaves inside them.
T1-weighted images are your anatomical roadmap. Fat looks bright white, and fluid like cerebrospinal fluid (CSF) is dark. This is the go-to for seeing brain structure-where the ventricles are, how the lobes line up, and whether there’s any swelling or mass pushing things out of place.
T2-weighted images flip the script. Water and fluid become bright white. That’s great for spotting edema, infection, or chronic damage. But here’s the catch: CSF is also bright on T2, which can make it hard to tell if a bright spot is a problem or just normal fluid. That’s where FLAIR comes in.
FLAIR (Fluid-Attenuated Inversion Recovery) is the unsung hero. It turns CSF dark while keeping abnormal tissue bright. This is critical for spotting multiple sclerosis plaques, which often cluster around the ventricles. Without FLAIR, those lesions could be hidden by the bright CSF glow. It’s also the best sequence for detecting small strokes or inflammation in the brain’s white matter.
Diffusion-weighted imaging (DWI) is the emergency responder. It picks up water that’s trapped-like in a stroke where brain cells die and can’t move fluid properly. If DWI shows a bright spot, it’s likely an acute stroke, even if the patient only had symptoms for 20 minutes. This changes everything: it can mean immediate clot-busting treatment. The key number? Apparent diffusion coefficient (ADC) values below 600 x 10^-6 mm²/s confirm acute ischemia.
Susceptibility-weighted imaging (SWI) is the blood detective. It picks up even the tiniest amounts of iron from old bleeds-like microhemorrhages from high blood pressure or amyloid angiopathy. These show up as dark dots, often in the brainstem or deep white matter. Finding them can help explain unexplained confusion or balance issues in older adults.
What Are the Most Common Findings?
Doctors don’t just look for dramatic abnormalities. Often, the most common findings are subtle-and sometimes normal for age.
White matter hyperintensities (WMHs) are bright spots on T2 or FLAIR images, usually near the ventricles. In people under 50, they’re uncommon. But by age 70, up to 90% of people have them. Most are harmless, linked to small vessel disease from high blood pressure or aging. But if they’re large, widespread, or appear suddenly, they might signal something more serious-like early vascular dementia or inflammation.
Small lacunar infarcts are tiny strokes, often under 5mm, caused by blocked deep arteries. They’re common in older adults with hypertension. You might not have had symptoms-they’re called “silent strokes.” But if there are several, especially in the basal ganglia or thalamus, it raises your risk of future stroke or cognitive decline.
Brain atrophy means shrinkage. Some loss of brain volume is normal with aging. But if the hippocampus (memory center) is shrinking faster than expected, it could point to Alzheimer’s. If the whole brain is shrinking rapidly, it might be linked to neurodegenerative diseases like Parkinson’s or frontotemporal dementia.
Incidental findings are surprises-things the doctor didn’t specifically look for. A small meningioma, a vestibular schwannoma (acoustic neuroma) as tiny as 2mm, or a benign cyst might show up. Most are harmless, but they need follow-up to make sure they’re not growing. That’s why radiologists check every part of the scan, including the cerebellopontine angle near the inner ear.
What Doesn’t MRI Show?
Even the best tool has limits. MRI can’t reliably tell you how old a lesion is without contrast or advanced techniques. A bright spot on FLAIR could be a recent stroke, an old one, or even inflammation from a past infection. That’s why doctors look at the bigger picture: your symptoms, blood tests, and sometimes repeat scans over time.
Also, MRI doesn’t show brain function. It shows structure. If you’re having memory problems, an MRI might show atrophy-but it won’t tell you if it’s Alzheimer’s, vascular dementia, or depression. For that, you need cognitive testing, PET scans, or sometimes spinal fluid analysis.
And while MRI is great for soft tissue, it’s terrible for bone. If you’ve had a fall and your doctor suspects a skull fracture, they’ll likely order a CT first. MRI might miss it entirely.
How Are Results Interpreted?
Reading a brain MRI isn’t random. Radiologists follow a strict system: start in the middle, move outward.
First, they check the ventricles-do they look too big? Is there asymmetry? Then they move to the basal ganglia and thalamus for small dark or bright spots. Next, the lobes: frontal, parietal, temporal, occipital. Are there any abnormal signals? Then the brainstem and cerebellum-often overlooked but critical for balance and coordination. Finally, the meninges and skull base, where tumors like schwannomas hide.
One common mistake? Mistaking blood vessels for lesions. Vessels have a natural dark signal called “flow void.” If you’re not trained, you might think it’s a hole in the brain. But experts know: if it’s a vessel, it will follow a clear path, not look like a blob.
Another pitfall: confusing normal age-related changes with disease. Periventricular hyperintensities are common in older adults. But if they’re symmetrical and in the right places, they’re likely benign. If they’re irregular, asymmetric, or spreading fast, that’s a red flag.

When Is an MRI Not Needed?
Not every headache or dizziness needs an MRI. In fact, for people with classic migraines and no neurological symptoms, guidelines from the American College of Radiology say MRI is “usually not appropriate.” Studies show only about 1.3% of brain MRIs done for simple headaches reveal anything serious.
Doctors use rules: if you have a sudden, worst headache of your life, vision loss, weakness, confusion, or seizures-MRI is essential. But if you’ve had the same dull headache for years, with no other symptoms, and your exam is normal, the risk of finding something dangerous is extremely low.
Overusing MRI doesn’t just cost money-it can lead to unnecessary worry. Finding a tiny, harmless cyst might send you down a path of repeated scans, biopsies, and anxiety. That’s why guidelines exist: to match the right test to the right patient.
What’s Next for Brain MRI?
Technology is moving fast. Ultra-high-field 7.0T MRI machines are now in some academic centers, showing brain layers so clearly you can see individual cortical bands. AI tools are cutting scan times in half without losing detail. And quantitative MRI-measuring things like myelin content or blood flow-is becoming routine in research and slowly making its way into clinics.
For now, though, the standard 1.5T or 3.0T machines are still the backbone of diagnosis. And while the cost can range from $1,200 to $3,500 in the U.S., the value is clear: catching a treatable condition early can change a life.
What matters most isn’t the machine-it’s the person interpreting it. A skilled radiologist doesn’t just see pixels. They see patterns. They know what’s normal for your age, your history, your symptoms. And they know when to say, “This is nothing,” or “This needs action.”
If you’ve just gotten your results, don’t panic. Many findings are normal. Many are treatable. And many-once understood-can be managed before they become serious. Ask your doctor to walk you through the images. Don’t just get a report. Understand what you’re looking at.
Can a brain MRI detect Alzheimer’s disease?
A brain MRI can’t diagnose Alzheimer’s directly, but it can show signs that support the diagnosis. The most common finding is shrinkage (atrophy) in the hippocampus and temporal lobes. While this isn’t unique to Alzheimer’s-other dementias cause similar changes-it helps rule out other causes like tumors or strokes. For a definitive diagnosis, doctors often combine MRI with cognitive tests and sometimes PET scans that detect amyloid plaques.
Why do I need to stay still during a brain MRI?
Even small movements-like swallowing or shifting your head-can blur the images. Brain MRI uses very precise magnetic fields to map tissue at the millimeter level. If you move, the computer can’t line up the images correctly, and important details might be missed. That’s why the scan takes 30-45 minutes: it needs time to capture multiple high-resolution sequences without motion artifacts.
Is it safe to have multiple brain MRIs over time?
Yes. Unlike CT or X-rays, MRI doesn’t use ionizing radiation. There’s no known risk from repeated scans. That’s why it’s the preferred method for monitoring conditions like multiple sclerosis, brain tumors, or epilepsy over years. The only concerns are metal implants or claustrophobia-not the scan itself.
What if my MRI shows white matter changes?
White matter changes are common, especially after age 50. They’re often linked to high blood pressure, aging, or small vessel disease. If they’re mild and stable, they usually don’t need treatment beyond managing blood pressure and cholesterol. But if they’re new, worsening, or located in unusual areas, your doctor may look for inflammation, MS, or other conditions. Location matters more than the amount.
Can MRI detect a brain tumor?
Yes, MRI is the best tool for detecting brain tumors. It can show their size, location, and how they’re affecting surrounding tissue. Tumors often appear as abnormal masses with irregular edges. Contrast dye may be used to see if the tumor is actively growing (it enhances with dye). MRI can also distinguish between benign and malignant tumors based on patterns of growth and invasion into nearby structures.
Do I need contrast dye for a brain MRI?
Not always. Many brain MRIs are done without contrast, especially for conditions like migraines, dementia, or stroke. Contrast (gadolinium) is used when doctors suspect active inflammation, infection, or a tumor that’s disrupting the blood-brain barrier. It helps highlight areas where the barrier is broken. If you have kidney problems, your doctor will check your function first-gadolinium is generally safe but requires caution in severe kidney disease.






Ashley Farmer
December 7, 2025 AT 05:58I had my first brain MRI last year after a weird episode of dizziness. Honestly, I was terrified they’d find something terrible. When the radiologist said it was just some normal aging stuff and tiny white matter changes, I cried with relief. It’s crazy how much anxiety these scans bring-even when they’re reassuring.
Just wanted to say: if you’re waiting on results, breathe. Most of what they see is just life happening inside your skull, not a death sentence.
Jennifer Anderson
December 7, 2025 AT 06:54omg i just got my mri results and i swear i thought i had a tumor lmao. turned out it was just some old tiny strokes i never even knew about. my doc said they’re called ‘silent’ because you don’t feel em. guess my high blood pressure’s been playing hide and seek with me for years.
also-why does everything on mri look like a ghost picture? like why is fluid white?? i need a meme guide to this.
Sadie Nastor
December 8, 2025 AT 15:14My grandma had a brain MRI last month and they found a 2mm cyst near her ear. She was convinced it was a spy drone. 😅
Turns out it’s a vestibular schwannoma-super common, totally harmless, and probably been there since she was 30. She’s now obsessed with watching YouTube videos of brain scans. I think she’s fallen in love with the FLAIR sequences. 🫶
Also, if you’re scared of the noise? Earplugs + Netflix on your phone during the scan = magic. They let you bring headphones in, y’all!
Sangram Lavte
December 9, 2025 AT 20:39As someone from India where access to MRI is expensive and delayed, I’ve seen how this tech saves lives. My cousin had a seizure last year-CT showed nothing. MRI found a small lesion in the temporal lobe. Surgery fixed it. No radiation, no guesswork.
But we need better awareness too. Too many people avoid scans because they think it’s only for ‘serious’ cases. It’s not. Early detection = better outcomes. Even if it’s just white matter changes, knowing helps you manage risk.
Also, don’t skip follow-ups. A ‘normal’ scan today doesn’t mean it’ll stay that way.
Oliver Damon
December 10, 2025 AT 11:07From a neuroimaging perspective, the real value of MRI lies in its ability to quantify microstructural integrity via DTI and quantitative mapping-techniques still underutilized in clinical practice. The T1/T2/FLAIR/DWI/SWI paradigm is foundational, but we’re moving toward voxel-wise myelin water fraction and perfusion-weighted imaging as biomarkers for neurodegeneration.
What’s often missed is that lesion load on FLAIR correlates more strongly with cognitive decline in aging than hippocampal volume alone. And yes-DWI is the gold standard for hyperacute stroke, but ADC thresholds vary by scanner field strength. 600 x 10⁻⁶ mm²/s is a heuristic, not an absolute.
Also, incidental findings >5mm warrant follow-up, but <3mm? Almost always benign. The anxiety cascade from incidentalomas is a public health issue in its own right.
Ernie Blevins
December 11, 2025 AT 11:36They’re lying to you. MRI doesn’t detect anything. It’s all just fancy shadows. The real cause of your symptoms? 5G towers, fluoride in the water, or Big Pharma pushing scans so they can sell you drugs. I had one and they said I had ‘white matter changes’-yeah, right. I don’t even have high blood pressure. They just want your money.
My cousin’s neurologist told him his brain looked ‘normal’-but he’s been sick for years. Coincidence? I think not.
David Brooks
December 12, 2025 AT 06:22Y’ALL. I just found out my 72-year-old dad has silent strokes. Like… five of them. And he’s been fine. Walking, cooking, telling bad jokes. I’m crying. Not because he’s sick-but because his brain is just… keeping going. Like a stubborn old engine.
Doctors said it’s from decades of high blood pressure. We’re starting him on meds, cutting salt, walking every day. I’m so proud of him. Brain scans aren’t scary-they’re a map to your body’s resilience. Don’t fear the scan. Fear not taking care of yourself.
Nicholas Heer
December 12, 2025 AT 17:23Why do they use magnets? I heard the government uses MRI to track your thoughts. That’s why they say it’s ‘safe’-because they’re not scanning your brain, they’re scanning your soul. And if you move? They get a glitch in the matrix.
My neighbor’s wife had an MRI and they found a ‘cyst.’ She died two months later. Coincidence? Nah. They didn’t tell her it was a ‘brain tumor’ because they don’t want you to panic. But you should panic. They’re hiding the truth.
Also-why do they always look at the cerebellopontine angle? That’s where the aliens hide.
Kurt Russell
December 14, 2025 AT 14:31Listen. If you’re reading this and you’re about to get an MRI-YOU GOT THIS. I’ve been through three. The machine sounds like a robot drumline, but you’re not trapped. You’re not alone. Breathe. Hum a song. Think of your favorite place.
And when you get the results? Don’t Google it. Go to your doctor. Ask them to point at the image and say, ‘This is normal. This is nothing. This is why we’re watching.’
You’re not broken. You’re being cared for. And that? That’s powerful.
Kyle Flores
December 14, 2025 AT 22:52My mom’s MRI showed WMHs and she started panicking. I printed out the whole paper and circled the part that said ‘common in >70yo’ and taped it to her fridge. She cried. Then she laughed.
Turns out she’s been walking 2 miles a day for 15 years and her BP’s perfect. Those spots? Just her brain’s history book. Not a warning sign.
So if you see bright spots? Don’t panic. Ask: ‘Is this new? Is it growing? Does it match my symptoms?’ If the answer’s no three times? It’s probably just your brain aging like fine wine.
Ryan Sullivan
December 16, 2025 AT 20:03It is axiomatic that the overutilization of neuroimaging in the absence of red flags constitutes a paradigmatic example of iatrogenic harm. The prevalence of incidental findings in asymptomatic populations exceeds 30% in cohort studies, leading to cascading diagnostic evaluations with negligible clinical benefit and substantial psychological morbidity.
Moreover, the conflation of radiological findings with clinical diagnosis represents a fundamental epistemic error in contemporary medical practice. The hippocampal volume reduction observed in aging is neither pathognomonic nor predictive of Alzheimer’s disease in isolation.
One must therefore invoke the principle of Occam’s razor: the most parsimonious explanation for a non-specific FLAIR hyperintensity is small vessel disease-not a neurodegenerative process.