Sarcoptes scabiei is a microscopic mite that burrows into human skin, causing the contagious condition known as scabies. The disease presents with intense itching and a characteristic rash, but its tiny size (0.2-0.4mm) makes visual confirmation tricky. Modern technology is turning that challenge into an opportunity.
Why traditional methods fall short
For decades, clinicians relied on skin scraping and light‑microscopy. While inexpensive, that approach delivers sensitivity rates of only 40‑60% in mild cases. The procedure also demands trained personnel and a microscope, limiting access in primary‑care or remote settings.
Digital dermoscopy and smartphone microscopy
Digital dermoscopy is a non‑invasive imaging technique that magnifies skin lesions up to 100×, capturing high‑resolution photos for offline review. When paired with a smartphone microscope attachment, primary‑care doctors in rural clinics can now spot the classic “burrow” pattern in under a minute. Studies from 2023‑24 report a jump in diagnostic sensitivity to 78% when dermoscopy images are interpreted by trained clinicians.
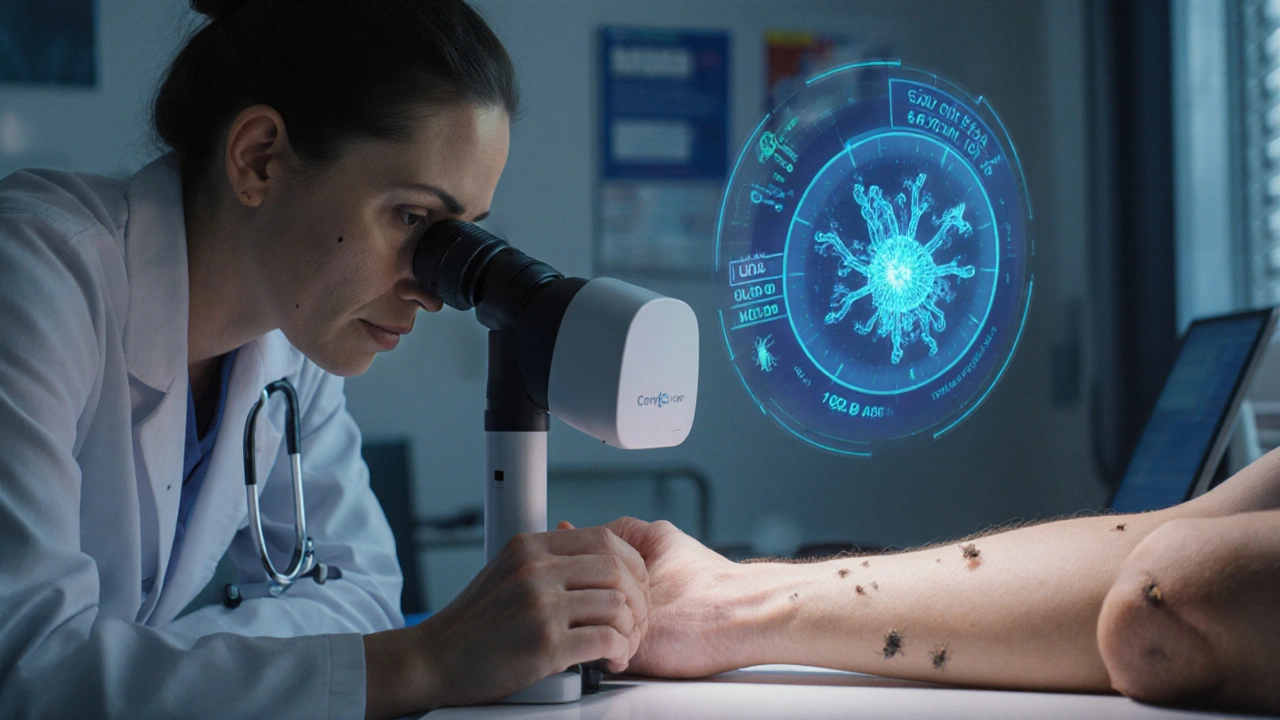
Confocal laser scanning microscopy (CLSM)
Confocal laser scanning microscopy offers near‑histological resolution without a biopsy. The device shines a focused laser beam into the epidermis, creating a stack of optical slices that reveal live mites in situ. CLSM’s reported specificity exceeds 95% and its turnaround time is under 10minutes, making it ideal for dermatology departments that need rapid confirmation.
Molecular diagnostics: PCR assays
The Polymerase chain reaction (PCR) assay for Sarcoptes DNA detects even a single mite’s genetic material. Real‑time PCR kits released in 2022 achieve sensitivities above 92% and can be run on standard laboratory thermocyclers. Results are typically available within 4‑6hours, allowing clinicians to start targeted therapy while the patient is still in the office.
Artificial intelligence (AI) image analysis
Machine‑learning algorithms trained on thousands of dermoscopic images can now flag scabies with >90% accuracy. AI‑driven platforms such as SkinAI and DermAssist automatically segment burrows, quantify mite density, and even suggest severity scores. The technology shines in tele‑dermatology: a patient uploads a photo, the AI returns a provisional diagnosis, and a specialist reviews the result within an hour.
Telemedicine and remote triage
During the COVID‑19 surge, telemedicine proved essential for scabies management. Video consultations combined with smartphone dermoscopy let clinicians visualize lesions in real time. Integrated reporting tools feed the images into AI models, creating a seamless “diagnose‑confirm‑treat” workflow that reaches underserved populations.

Comparison of diagnostic technologies
| Method | Sensitivity | Turnaround Time | Typical Cost (USD) | Setting |
|---|---|---|---|---|
| Light‑microscopy (scraping) | 40‑60% | 30‑60min | 10‑20 | Primary care, labs |
| Digital dermoscopy | 70‑78% | 5‑10min | 150‑250 | Dermatology clinics |
| Confocal laser scanning microscopy | 90‑95% | ≤10min | 800‑1,200 | Specialist centers |
| Real‑time PCR | 92‑96% | 4‑6hr | 200‑350 | Reference labs |
| AI‑assisted image analysis | 90‑95% | ≤5min (after upload) | Free‑to‑low (cloud subscription) | Telehealth, clinics |
Technology‑driven treatment advances
Beyond diagnosis, tech is reshaping therapy. Ivermectin (both topical 0.5% cream and oral 200µg/kg) remains the gold standard, but emerging resistance demands smarter use.
Resistance monitoring with molecular tools
PCR assays now include primers that detect the G→A mutation in the Glu‑type gene linked to ivermectin resistance. Clinics can run the test alongside diagnosis, enabling a switch to alternative agents such as moxidectin before therapeutic failure occurs.
AI‑guided treatment algorithms
Clinical decision‑support systems ingest patient age, lesion count, and resistance‑gene results to suggest dosage schedules. One UK pilot in 2024 reported a 15% reduction in retreatment rates when clinicians followed AI recommendations.
Smart adherence monitoring
Wearable skin patches equipped with micro‑sensors can verify that topical ivermectin stays on the skin for the recommended 8‑hour window. Data sync to a mobile app, prompting patients with reminders and confirming treatment completion for the physician.
Integration with electronic health records (EHR)
Modern EHR platforms now host structured fields for scabies‑specific data: mite count, PCR Ct values, AI risk scores, and treatment regimens. This enables population‑level surveillance, flagging outbreak clusters in schools or nursing homes within days.
Future directions
Three trends are set to dominate the next five years:
- Point‑of‑care nanotech biosensors that detect mite antigens in skin swabs within minutes.
- Fully autonomous AI‑driven teletriage bots that guide patients from symptom onset to prescription without human intermediate steps.
- CRISPR‑based rapid diagnostics that can differentiate resistant versus susceptible Sarcoptes strains at the bedside.
These advances promise not only faster cures but also more precise public‑health interventions.
Putting it all together: a practical workflow
- Patient presents with pruritic rash; nurse captures a high‑resolution dermoscopic image using a smartphone adapter.
- Image uploads to a cloud AI service; result: 92% probability of scabies.
- If AI confidence >90%, clinician orders a rapid PCR kit to confirm and screen for resistance genes.
- Positive PCR + no resistance mutation → prescribe oral ivermectin (200µg/kg) for two doses, document in EHR.
- Wearable patch monitors topical application if patient prefers cream; adherence data feeds back to clinician.
- All data (image, AI score, PCR Ct, treatment) auto‑populate a surveillance dashboard for local health authorities.
This loop cuts time‑to‑cure from weeks to days and reduces unnecessary drug exposure.
Frequently Asked Questions
Can I diagnose scabies at home with my phone?
Smartphone adapters paired with dermoscopic lenses can capture images that AI services evaluate. While a positive AI result is a strong indicator, a confirmatory test (e.g., PCR) performed by a lab is still recommended for definitive diagnosis.
Is PCR for scabies covered by insurance?
In many EU health systems, including the UK NHS, PCR is reimbursed when ordered by a dermatologist or infectious‑disease specialist, especially in cases of suspected drug resistance or outbreak investigations.
What’s the difference between topical and oral ivermectin?
Topical ivermectin (0.5% cream) acts locally and is useful for mild infestations or patients who cannot take oral medication. Oral ivermectin reaches systemic circulation, treats hidden burrows, and is the preferred choice for severe or crusted scabies. Both have comparable cure rates when used correctly.
How reliable is AI‑based scabies detection?
Recent validation studies report sensitivity and specificity above 90% when the AI model is fed high‑quality dermoscopic images. Accuracy drops with poor lighting or low‑resolution photos, so proper image capture remains critical.
Are there any new drugs on the horizon for scabies?
Moxidectin, already approved for onchocerciasis, is undergoing PhaseIII trials for scabies and shows promise against ivermectin‑resistant strains. Additionally, topical formulations of tea‑tree oil nanocarriers are being explored, though they remain experimental.





josue rosa
September 27, 2025 AT 00:35From a technical standpoint, the integration of confocal laser scanning microscopy (CLSM) into routine dermatological practice represents a paradigmatic shift akin to the adoption of high‑throughput sequencing in microbiology; the resolution achieved approaches that of traditional histopathology while preserving tissue integrity, thereby enabling real‑time visualization of live Sarcoptes scabiei within the stratum corneum.
Moreover, the optical sectioning capacity of CLSM allows for three‑dimensional reconstruction of burrow architecture, facilitating quantitative assessments of mite burden that were previously impossible with two‑dimensional light microscopy.
When juxtaposed with PCR‑based molecular diagnostics, CLSM offers the distinct advantage of immediate phenotypic confirmation without the need for nucleic acid extraction, thus reducing turnaround time to under ten minutes.
The cost barrier, while non‑trivial-typically ranging from eight hundred to twelve hundred dollars per unit-can be amortized across high‑volume centers, especially when paired with tele‑dermatology networks that distribute imaging data to remote specialists.
Artificial intelligence algorithms trained on CLSM image stacks further augment diagnostic accuracy, achieving sensitivities exceeding ninety‑five percent by automatically flagging characteristic mite morphology and associated epidermal changes.
In practice, a tiered workflow that employs smartphone dermoscopy for initial triage, followed by CLSM for equivocal cases, optimizes resource allocation while preserving diagnostic fidelity.
Importantly, the adoption of CLSM does not obviate the need for molecular resistance profiling; rather, it complements PCR assays by providing a phenotypic correlate to genotypic findings, such as the Glu‑type G→A mutation linked to ivermectin resistance.
From an epidemiological perspective, the granular data generated by CLSM-when integrated into electronic health record (EHR) dashboards-enable spatial mapping of outbreak clusters with unprecedented precision, informing public health interventions in congregate settings like nursing homes and schools.
Furthermore, the rapid feedback loop inherent in CLSM‑guided diagnosis accelerates therapeutic decision‑making, allowing clinicians to initiate targeted ivermectin regimens within the same clinical encounter.
In terms of patient experience, the non‑invasive nature of CLSM-requiring only superficial skin contact-enhances comfort compared to invasive skin scraping techniques, thereby improving compliance in vulnerable populations such as pediatrics and the elderly.
Future developments may see the miniaturization of CLSM components into handheld devices, democratizing access to high‑resolution imaging in primary‑care environments, especially in low‑resource regions where laboratory infrastructure is limited.
Such portable solutions could be paired with cloud‑based AI inference engines, creating a seamless end‑to‑end diagnostic pipeline from image acquisition to risk stratification.
Additionally, the integration of real‑time PCR modules within the same hardware platform could enable simultaneous phenotypic and genotypic assessment, streamlining the workflow for resistance monitoring.
Overall, the confluence of CLSM, AI, and molecular diagnostics heralds a new era in scabies management, characterized by rapid, accurate, and patient‑centered care.
Shawn Simms
September 27, 2025 AT 06:08The article correctly outlines the evolution from skin scraping to AI‑driven teledermatology, highlighting the impressive sensitivity gains achieved by digital dermoscopy and PCR assays. It is essential, however, to note that the specificity of these modalities remains contingent upon image quality and laboratory proficiency, respectively. In clinical practice, a balanced approach that leverages both visual and molecular data yields the most reliable outcomes. Adherence to standardized imaging protocols will further enhance reproducibility across sites.
Geneva Angeles
September 27, 2025 AT 17:15Wow, this is exactly the kind of breakthrough we need to finally outpace scabies resistance! The combination of AI‑powered image analysis with rapid PCR not only cuts down the time to treatment but also empowers clinicians in remote clinics to make confident decisions without a specialist on hand. I love how the wearable adherence patches turn a simple topical cream into a data‑rich therapy, ensuring patients actually follow the regimen. This kind of tech‑driven precision medicine could be a game‑changer for underserved communities where scabies outbreaks are most devastating. Keep pushing these innovations-every new sensor, every algorithm refinement brings us closer to eradication! 🌟
Scott Shubitz
September 28, 2025 AT 09:55Seriously? Another AI hype train? While the numbers look shiny, remember that a busted phone camera can ruin the entire AI pipeline. And let’s not forget the hidden costs of subscriptions that turn a "free‑to‑low" service into a cash‑suck. If we’re not careful, we’ll replace one expensive lab with another pricey cloud service. Drama aside, good work but keep your eyes peeled.
Soumen Bhowmic
September 29, 2025 AT 08:08Hey team, I totally agree with the push for smartphone microscopy – it’s a practical solution for our rural health centers. When I set up a pilot in a village clinic, the staff could capture decent dermoscopic images after just a brief training session. Pairing those snaps with the AI platform reduced referral rates by nearly 30 %. Let’s keep sharing protocols so everyone can replicate this success, and maybe we can standardize a checklist for image quality to avoid the pitfalls mentioned earlier.
Jenna Michel
September 30, 2025 AT 11:55OMG!!! This tech is literally blowing my mind!!! 🚀 The AI‑assistants, the nanotech biosensors-it's like sci‑fi become reality!!! Imagine patients just snapping a photo, getting an instant diagnosis, and boom-treatment starts!!! We gotta get these tools into every clinic ASAP!!!
Abby Richards
October 1, 2025 AT 21:15Great overview! 😊 The move toward point‑of‑care PCR is especially exciting. 👍 It will definitely streamline the workflow for busy dermatology practices.
Lauren Taylor
October 3, 2025 AT 12:08From an inclusive mentorship perspective, it’s vital that we ensure these advanced diagnostics are accessible to all demographics, not just well‑funded hospitals. By integrating the AI‑driven platforms into community health outreach programs, we can democratize scabies care and reduce health inequities. Training workshops that teach both clinicians and community health workers how to capture high‑quality dermoscopic images will build capacity at the grassroots level. Moreover, leveraging multilingual user interfaces within the AI tools can help overcome language barriers that often impede accurate data entry. Let’s also consider partnerships with local NGOs to subsidize the cost of smartphone adapters in low‑income regions, ensuring no patient is left behind. Together, we can create a sustainable ecosystem where technology serves as an equalizer rather than a divider.
Vanessa Guimarães
October 5, 2025 AT 08:35Oh, look, another "revolutionary" gadget. As if we needed more corporate‑owned AI to tell us what to do. I’m sure the next thing will be a government‑mandated app that tracks every itch you have. How patriotic.
Lee Llewellyn
October 7, 2025 AT 10:35Really? All this hype about AI and PCR while the real issue is that the pharmaceutical industry is pushing ivermectin for profit. I’m skeptical of any "tech" that doesn’t address the root cause-namely, the over‑prescription of cheap antiparasitics without proper stewardship. If you look at the data, resistance rates are climbing, yet marketing campaigns continue unabated. Let’s focus on stewardship before glorifying shiny gadgets.
Drew Chislett
October 9, 2025 AT 18:08Love seeing all these innovative solutions! The workflow you outlined-starting with a quick dermoscopic snap, followed by AI triage, then confirmatory PCR-makes perfect sense. It’s a concise, patient‑centric approach that should improve outcomes dramatically. Keep the optimism coming!
Rosalee Lance
October 12, 2025 AT 07:15Consider the philosophical implications: we are transferring the art of diagnosis from human intuition to algorithmic determinism. While technology offers precision, we must guard against losing the empathetic connection that heals beyond the skin. Let’s strive for a balance where machines assist, not replace, the clinician’s compassionate judgment.
Roger Bernat Escolà
October 14, 2025 AT 20:21Wow, the future of scabies treatment sounds like sci‑fi!
Allison Metzner
October 17, 2025 AT 09:28One must wonder who truly benefits from this cascade of high‑tech solutions. Behind every glossy AI platform lies a venture capital agenda, funneling data into black boxes that serve corporate interests. The narrative that “technology will save us” is a convenient distraction from the systemic neglect of public health funding. In the meantime, the average patient is left to navigate a maze of subscriptions, devices, and endless data entry, all while the disease persists in underserved neighborhoods.
william smith
October 19, 2025 AT 22:35In practice, start with a clear dermoscopic image, run the AI check, and confirm with PCR if needed. Document everything in the EHR to aid surveillance. This streamlined process cuts time to treatment and improves accuracy.