When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how do regulators know it’s truly the same? The answer lies in a quiet but powerful standard: the 80-125% rule. It’s not about how much active ingredient is in the pill. It’s not about taste, color, or price. It’s about what happens inside your body after you swallow it.
What the 80-125% Rule Actually Means
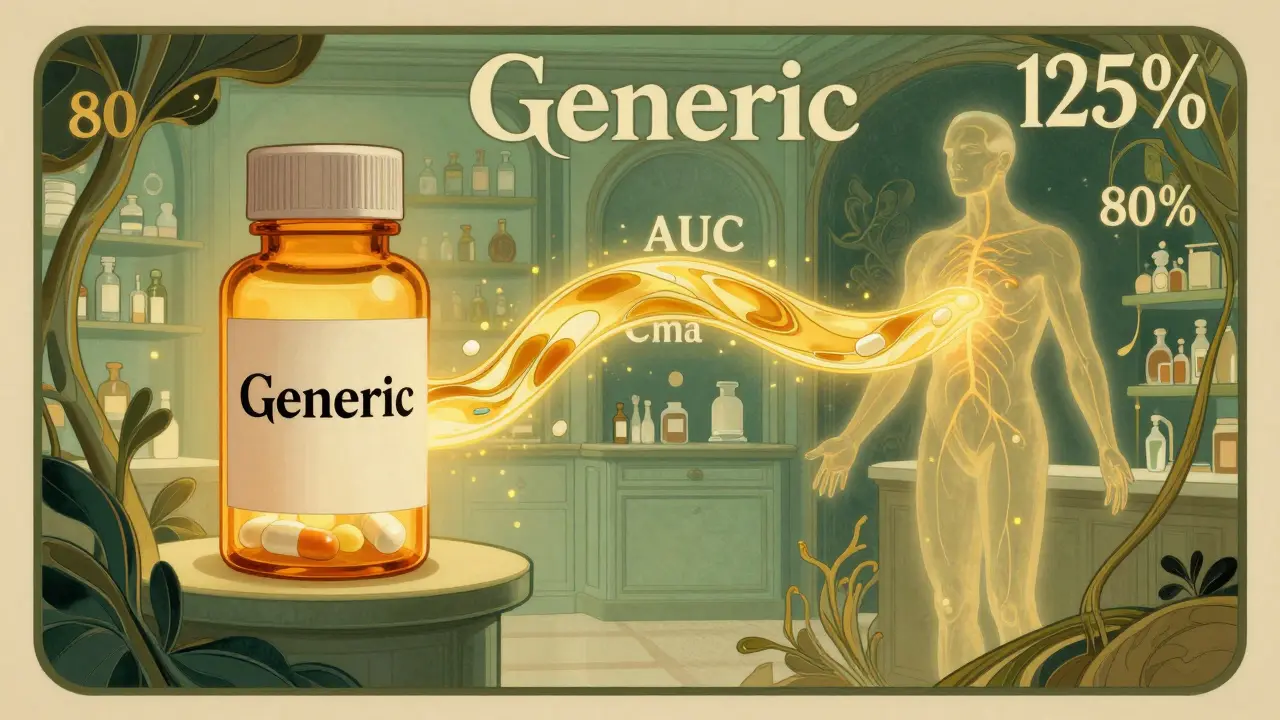
Many people think the 80-125% rule means generic drugs can contain anywhere from 80% to 125% of the active ingredient compared to the brand name. That’s wrong. A generic drug must contain the same amount of active ingredient as the original - usually within 95% to 105% of the labeled dose. The 80-125% rule applies to something far more complex: how quickly and completely your body absorbs that drug.
Regulators measure two key things: AUC (Area Under the Curve), which tells you how much of the drug enters your bloodstream over time, and Cmax, which shows the highest concentration reached. These aren’t guesses. They’re precise measurements taken from clinical studies where healthy volunteers take both the brand and generic versions under controlled conditions.
The rule says that the 90% confidence interval of the ratio between the geometric means of these two values (generic vs. brand) must fall entirely between 80% and 125%. That’s it. If even one point of that interval dips below 80% or goes above 125%, the drugs aren’t considered bioequivalent - and the generic can’t be approved.
Why Logarithms and Confidence Intervals?
Why not just compare average numbers directly? Because drug absorption doesn’t follow a normal pattern. It’s skewed. One person might absorb a drug slowly, another rapidly. That’s why scientists use logarithmic transformation. On a log scale, a 20% difference above and below becomes symmetrical. An AUC ratio of 80% equals -0.2231 on the log scale. A ratio of 125% equals +0.2231. The math works cleanly.
And why a 90% confidence interval instead of the more familiar 95%? Because regulators are okay with a 5% chance of error on each end - meaning there’s a 10% total chance the conclusion could be wrong. That’s a deliberate trade-off. It’s stricter than most statistical tests because the goal isn’t to prove the drugs are identical - it’s to prove they’re close enough that no patient will notice a difference in effect or side effects.
It’s Not Just the FDA - It’s Global
The 80-125% rule isn’t an American quirk. It’s the global gold standard. The European Medicines Agency (EMA), the World Health Organization (WHO), Health Canada, China’s NMPA, and dozens of other agencies all use it. This isn’t coincidence. It’s by design. Harmonization lets drug companies develop one set of data and get approval in multiple countries at once. Without this rule, the generic drug market would be a patchwork of conflicting standards - and cheaper medicines wouldn’t be available so widely.
That’s why over 14,000 generic drugs have been approved in the U.S. alone since 1984. Today, 90% of prescriptions in the U.S. are filled with generics - but they make up only 23% of total drug spending. That’s billions saved every year. The 80-125% rule made that possible.

When the Rule Isn’t Enough
Not all drugs play nice with the 80-125% rule. Some are too variable. Others are too dangerous to risk even small differences.
Take narrow therapeutic index (NTI) drugs - medications where a tiny change in blood level can cause harm or failure. Warfarin, levothyroxine, phenytoin, and cyclosporine fall into this category. For these, regulators have tightened the limits. The FDA now recommends a 90-111% range for certain NTI drugs. The EMA allows it too. That’s a much smaller window - and for good reason. A 20% drop in levothyroxine could leave a patient hypothyroid. A 20% spike in warfarin could cause internal bleeding.
Then there are highly variable drugs. These are drugs where the same person’s absorption varies wildly from one dose to the next - often due to metabolism differences. For these, the 80-125% rule can be too strict. That’s where scaled average bioequivalence (SABE) comes in. If a drug’s within-subject variability is over 30%, regulators can expand the range - up to 69.84-143.19% for Cmax - but only if the data supports it. This isn’t a loophole. It’s a scientifically adjusted tool for tricky cases.
Common Misconceptions
Even among pharmacists and doctors, confusion runs deep. A 2022 survey found 63% of community pharmacists thought the 80-125% rule meant the active ingredient content varied that much. It doesn’t. The active ingredient is tightly controlled. The rule applies only to absorption - how fast and how much gets into your blood.
Patients worry too. Online forums are full of stories: “My generic seizure med didn’t work.” “I had side effects after switching.” These are real concerns. But the data tells a different story. A 2020 FDA analysis of over 2,000 generic drugs approved between 2003 and 2016 found only 0.34% required label changes due to bioequivalence issues after they hit the market.
Most problems with generics aren’t caused by the 80-125% rule. They’re caused by inactive ingredients - fillers, dyes, coatings - that trigger allergies or affect how the pill breaks down. Or by switching between different generic manufacturers. The rule doesn’t guarantee identical performance across all generics - only that each one is equivalent to the original brand.
How Studies Are Done
Getting a generic approved isn’t easy. A typical bioequivalence study involves 24 to 36 healthy volunteers. Each person takes both the brand and generic versions in a randomized order, separated by a washout period. Blood samples are taken every 15 to 30 minutes for up to 72 hours. Then the data is analyzed using strict statistical models.
It’s not just about the numbers. Regulators also check: Did the study follow Good Clinical Practice? Were outliers handled properly? Was food taken into account? For drugs that interact with food, studies must be done both fasted and after a meal. If the drug is modified-release - like a slow-dissolving tablet - the study design gets even more complex.
And it’s expensive. Bioequivalence studies cost between $2 million and $5 million and take 18 to 24 months. That’s why only serious companies invest in generics. The payoff? A huge market. In 2023, the global generic drug market was worth $227 billion.
What’s Next?
The 80-125% rule has held up for nearly 40 years. But science is moving. The FDA is investing $15 million to explore model-informed bioequivalence - using computer simulations and population data instead of always relying on human trials. For complex products like inhalers, injectables, and topical creams, traditional methods often don’t work. New tools are needed.
Researchers are also looking at pharmacogenomics. What if your genes affect how you absorb a drug? Could future bioequivalence standards consider genetic profiles? That’s still years away - but it’s being studied.
For now, the rule remains the backbone of generic drug approval. It’s not perfect. It’s not always intuitive. But it works. Millions of people take generics every day without issue. And that’s because regulators didn’t just pick 80 and 125 out of thin air. They built it on decades of data, clinical experience, and statistical rigor.
Does the 80-125% rule mean generic drugs are weaker than brand-name drugs?
No. The rule doesn’t refer to the amount of active ingredient in the pill. Generics must contain the same amount as the brand - typically within 95% to 105% of the labeled dose. The 80-125% range applies only to how much of the drug enters your bloodstream (AUC) and how fast it gets there (Cmax), based on clinical study data. It’s about absorption, not dosage.
Why is a 90% confidence interval used instead of 95%?
A 90% confidence interval allows for a 5% risk of error on each side - totaling a 10% risk of concluding two drugs are equivalent when they’re not. This is intentional. Regulatory agencies prioritize practical safety over statistical conservatism. A 95% interval would require even tighter bioequivalence, making it harder to approve generics without adding clinical benefit. The 90% level strikes a balance between scientific rigor and real-world usability.
Are all generic drugs required to meet the 80-125% rule?
Almost all. For standard immediate-release oral tablets and capsules, yes. But exceptions exist. For narrow therapeutic index drugs like warfarin or levothyroxine, stricter limits (like 90-111%) apply. For highly variable drugs, regulators may use scaled bioequivalence methods that allow wider ranges. Complex products like inhalers, patches, or extended-release formulations may follow different criteria based on their delivery system.
Can a generic drug pass bioequivalence testing but still cause side effects?
Yes. Bioequivalence ensures similar absorption rates and total exposure, but it doesn’t control for inactive ingredients. Differences in fillers, dyes, or coatings can trigger allergies, affect how quickly the pill dissolves, or change how it’s absorbed in the gut. That’s why some patients report different experiences when switching between generic brands - even if both meet the 80-125% rule. It’s not a failure of the rule; it’s a limitation of what it measures.
How often do generic drugs fail bioequivalence testing?
It’s rare, but it happens. Most failures occur during early development, before submission. Once a generic is approved, post-market surveillance shows only 0.34% of approved generics required label changes due to bioequivalence concerns between 2003 and 2016. Many failed studies never reach the market - companies often stop development if early data looks poor. The system is designed to catch problems before patients are affected.






srishti Jain
December 31, 2025 AT 00:29This rule is such a scam. I switched generics and my anxiety went through the roof. They don't care if you suffer as long as the numbers look good.
Aayush Khandelwal
January 1, 2026 AT 19:57Let’s unpack this with some pharmacokinetic granularity. The 80-125% CI isn’t some arbitrary fairy-tale number-it’s the sweet spot where geometric mean AUC and Cmax ratios, log-transformed and normalized via within-subject variability, converge into a clinically inert zone. It’s Bayesian pragmatism wrapped in frequentist packaging. The FDA didn’t pull this from a hat-they crunched decades of population PK data from 12,000+ trials. This is peak regulatory elegance.
Colin L
January 3, 2026 AT 12:14Look, I get the theory, but I’ve been on the same generic for five years and my doctor just switched me to another one-same active ingredient, same 80-125% compliance-and suddenly I’m dizzy, nauseous, and my heart’s racing. I’m not imagining this. The system says it’s fine, but my body says otherwise. And no, I don’t have time to go through 12 different brands until I find the one that doesn’t make me feel like I’m dying. What’s the point of a ‘safe’ standard if it doesn’t keep people safe? You’re talking math while I’m talking survival.
And don’t even get me started on how some generics use different fillers that trigger my histamine response. I’m not a lab rat. I’m a human being with a gut that reacts to corn starch like it’s poison. Who approved that? The FDA? The WHO? Some guy in a suit who’s never had to take this stuff daily for a decade?
It’s not just bioequivalence-it’s biocompatibility. And nobody’s measuring that. Not really. Not in the real world.
Hayley Ash
January 3, 2026 AT 20:15Oh wow the 80-125 rule is sooo scientific wow I'm so impressed how many graphs they had to draw to justify letting drug companies save billions while we pay the price in side effects
Also why is it always healthy volunteers? Ever think maybe your 80-year-old diabetic grandma absorbs drugs differently than a 22-year-old gym bro? Nah, let's just assume everyone's the same
And don't get me started on how the FDA lets them skip food-effect studies if they're 'convenient' hahaha
kelly tracy
January 4, 2026 AT 17:52Stop pretending this is science. This is corporate lobbying dressed up as statistics. The 80-125% rule was created to let Big Pharma outsource manufacturing to the cheapest country possible while pretending patients won’t notice the difference. And they’re right-most people won’t. But some of us do. And we’re the ones getting ignored. The FDA approves generics like they’re cereal boxes. One batch passes, the next batch fails, but you still get it on the shelf because ‘it’s within range.’ That’s not medicine. That’s gambling with your life.
And don’t tell me about the 0.34% failure rate. That’s just the ones they caught. How many people got sick and never reported it? How many died quietly because no one cared enough to connect the dots?
Cheyenne Sims
January 4, 2026 AT 20:43The 80-125% bioequivalence criterion is a scientifically validated, internationally harmonized standard developed through rigorous clinical pharmacokinetic analysis. It is not a loophole, nor is it a compromise-it is a precisely calibrated threshold based on decades of empirical evidence. To suggest otherwise reflects a fundamental misunderstanding of pharmacological regulation and statistical methodology. The U.S. Food and Drug Administration adheres to the highest international standards, and the approval process for generic pharmaceuticals is among the most stringent in the world. Any anecdotal claims regarding adverse effects are not indicative of systemic failure but rather isolated cases potentially attributable to non-pharmacological variables such as excipient sensitivity or medication adherence.
Shae Chapman
January 6, 2026 AT 03:01I just want to say thank you for explaining this so clearly 😊 I’ve been on levothyroxine for 10 years and switched generics 3 times and thought I was crazy for feeling off every time. Now I get it-it’s not the drug, it’s the fillers. I’ll start asking my pharmacist for the same manufacturer every time. Also, the part about SABE for highly variable drugs? Mind blown 🤯 I never knew regulators had that flexibility. This post made me feel less alone and way more informed. Thank you for doing the deep dive 💪❤️
Nadia Spira
January 6, 2026 AT 05:29You think this is about science? Nah. This is about control. The 80-125% rule is a performative illusion of rigor-engineered to pacify the public while the real power lies in who funds the studies, who owns the patents, and who gets to decide what ‘clinically equivalent’ means. They use jargon like ‘geometric mean’ and ‘within-subject variability’ to bury the truth: the system is designed to protect profits, not patients. You don’t need a PhD to see that. You just need to ask: Who benefits? And who pays the price?
And don’t give me that ‘90% of prescriptions are generics’ nonsense. That’s not progress-it’s commodification. You turn medicine into a commodity, and people become variables in a spreadsheet. Wake up.